研究内容
研究の方針
私たちの研究は、遷移金属触媒または外部刺激(光など)によって促進される新反応の開発、およびそれらを可能にする配位子と試薬の合理的な選択と設計に基づいています。これらの新反応の開発を通じて、私たちは有機合成における長年の制約と課題を克服し、有用な未踏分子を創製しています。これらの研究では、実験だけでなく理論計算も重要です。理論計算は、反応機構や物性を解釈するだけでなく、反応経路や物性を予測するための強力な手法となります。以下に、これまでに報告した研究の例を示します。
✓ Annulation reactions✓ σ-bond activation reactions
✓ Π-bond activation reactions
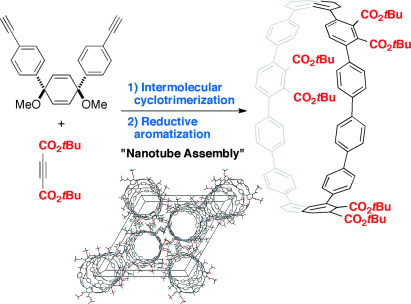
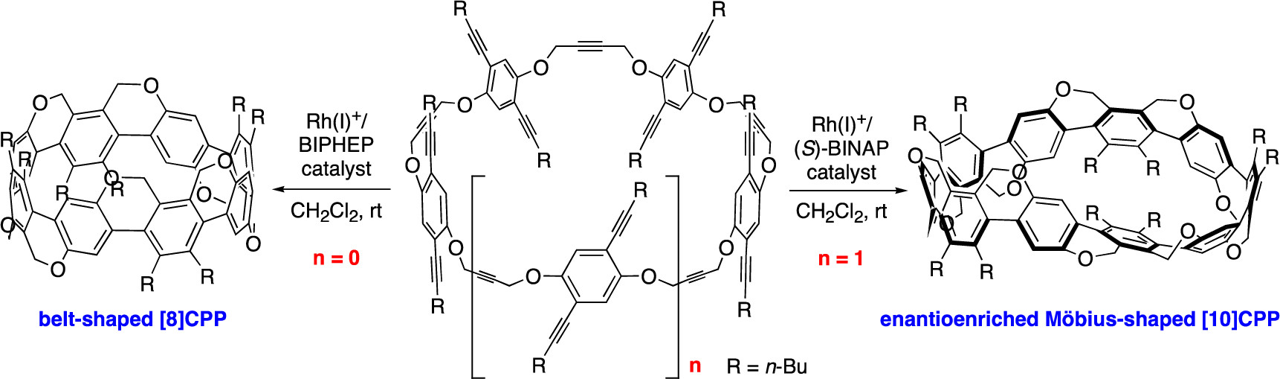
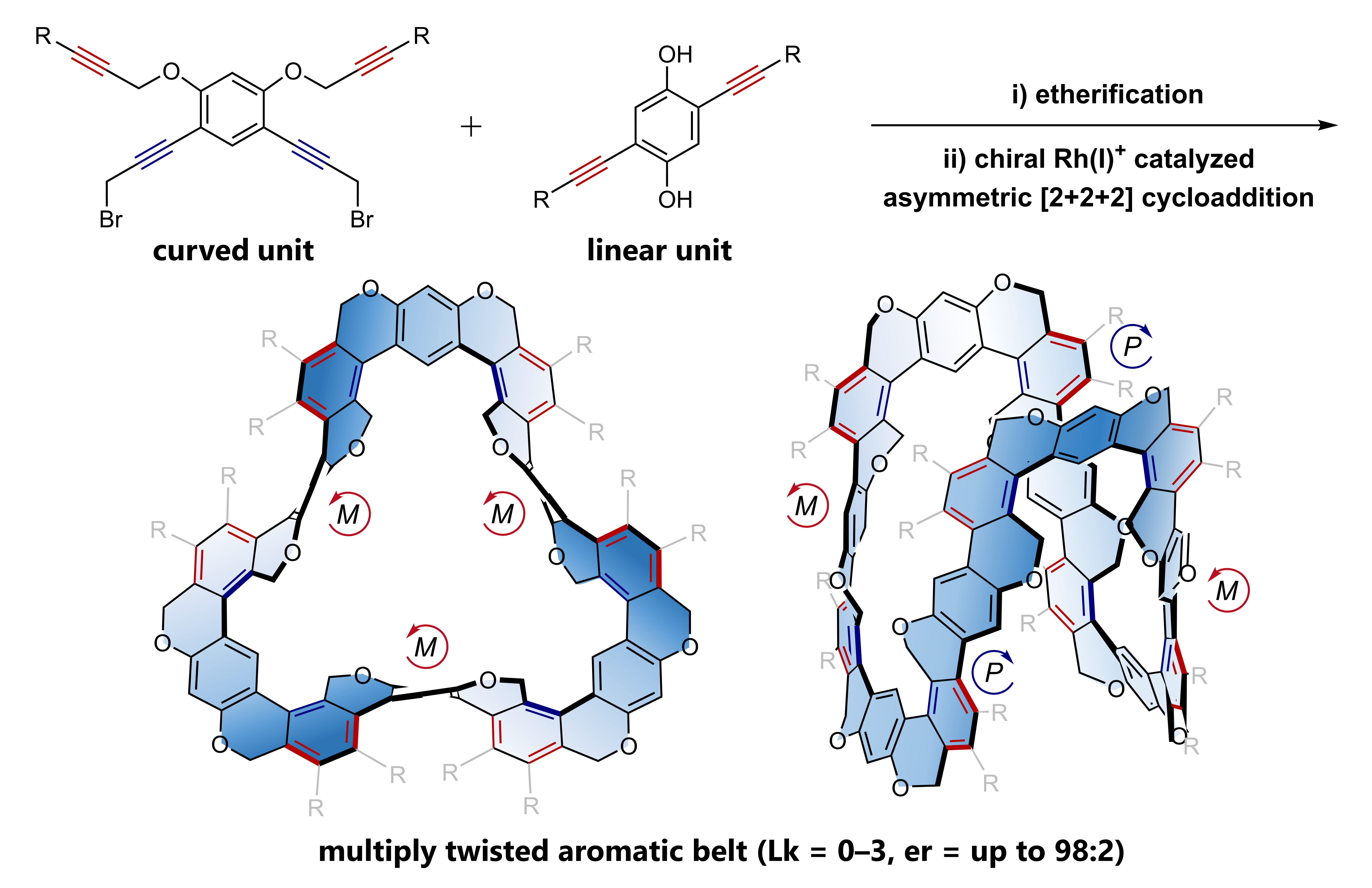
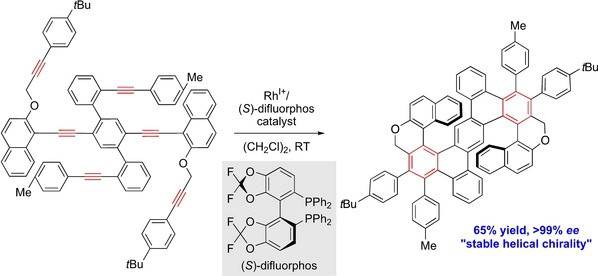
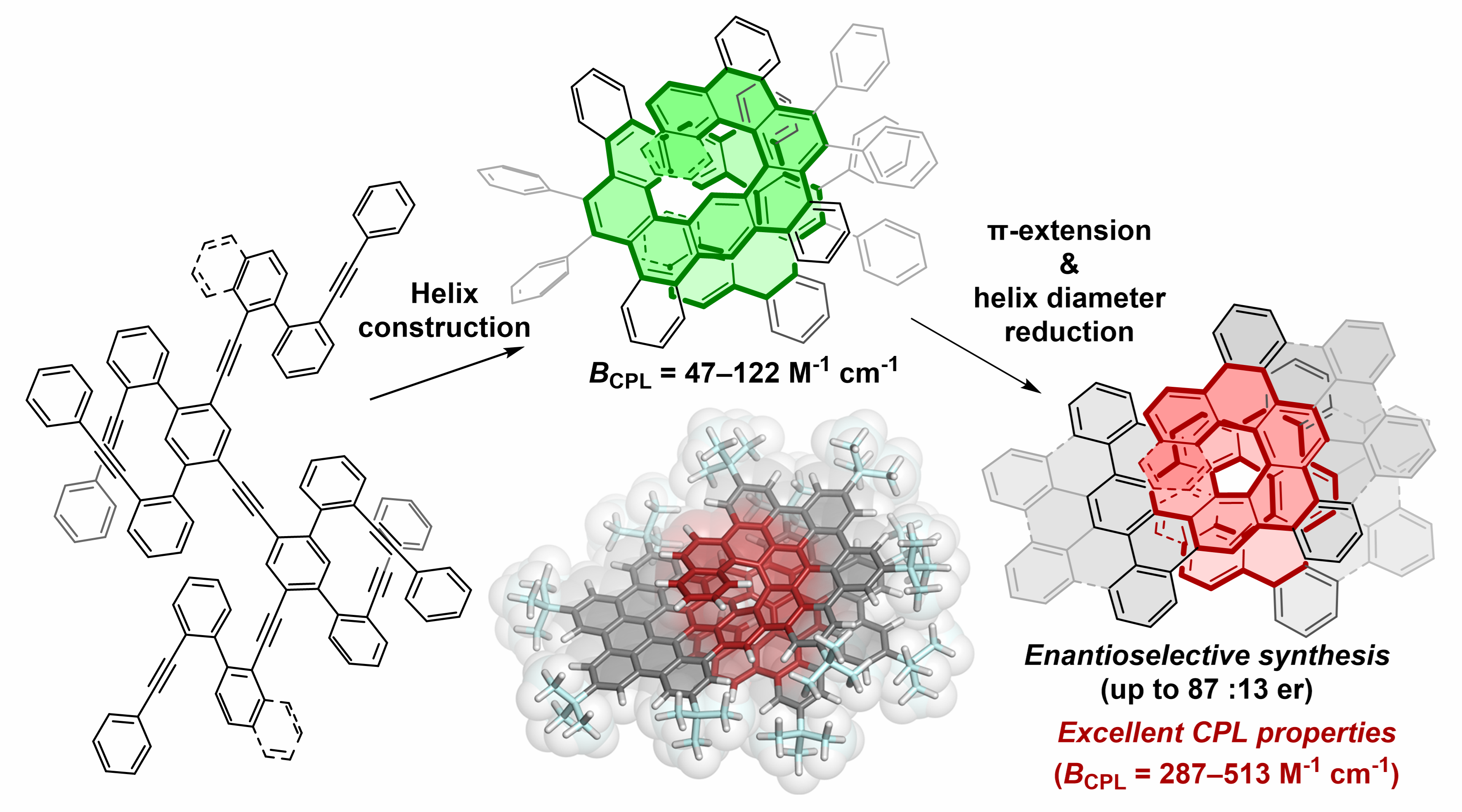
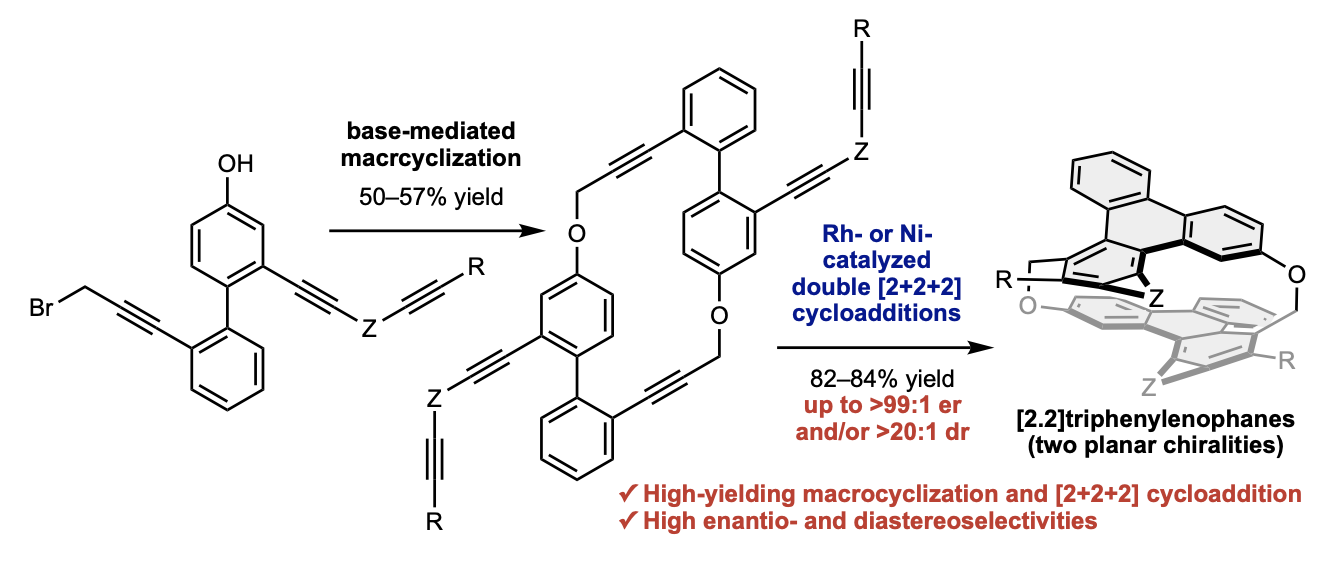
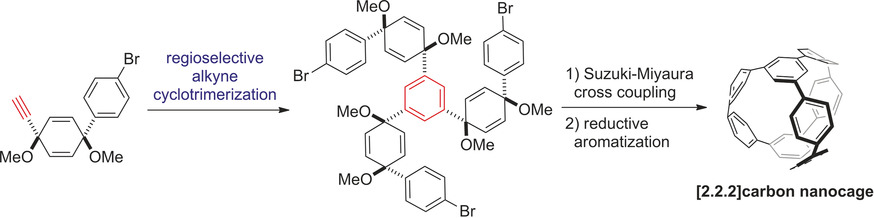
✓ 3D Nanocarbon synthesis by [2+2+2] cycloadditions
Carbon nanorings / Single and multi-helicenes / Cyclophanes / Cages
✓ Synthesis of novel azahelicenes
Annulation reactions
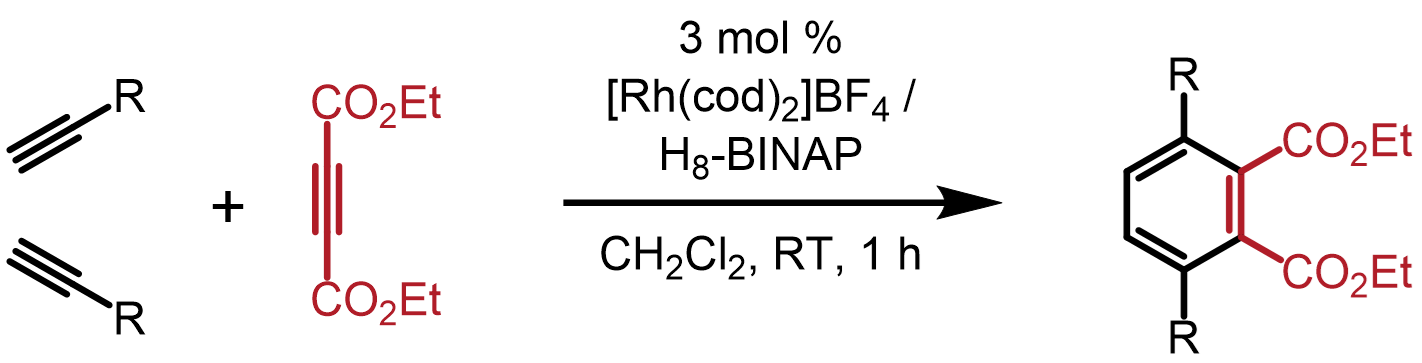
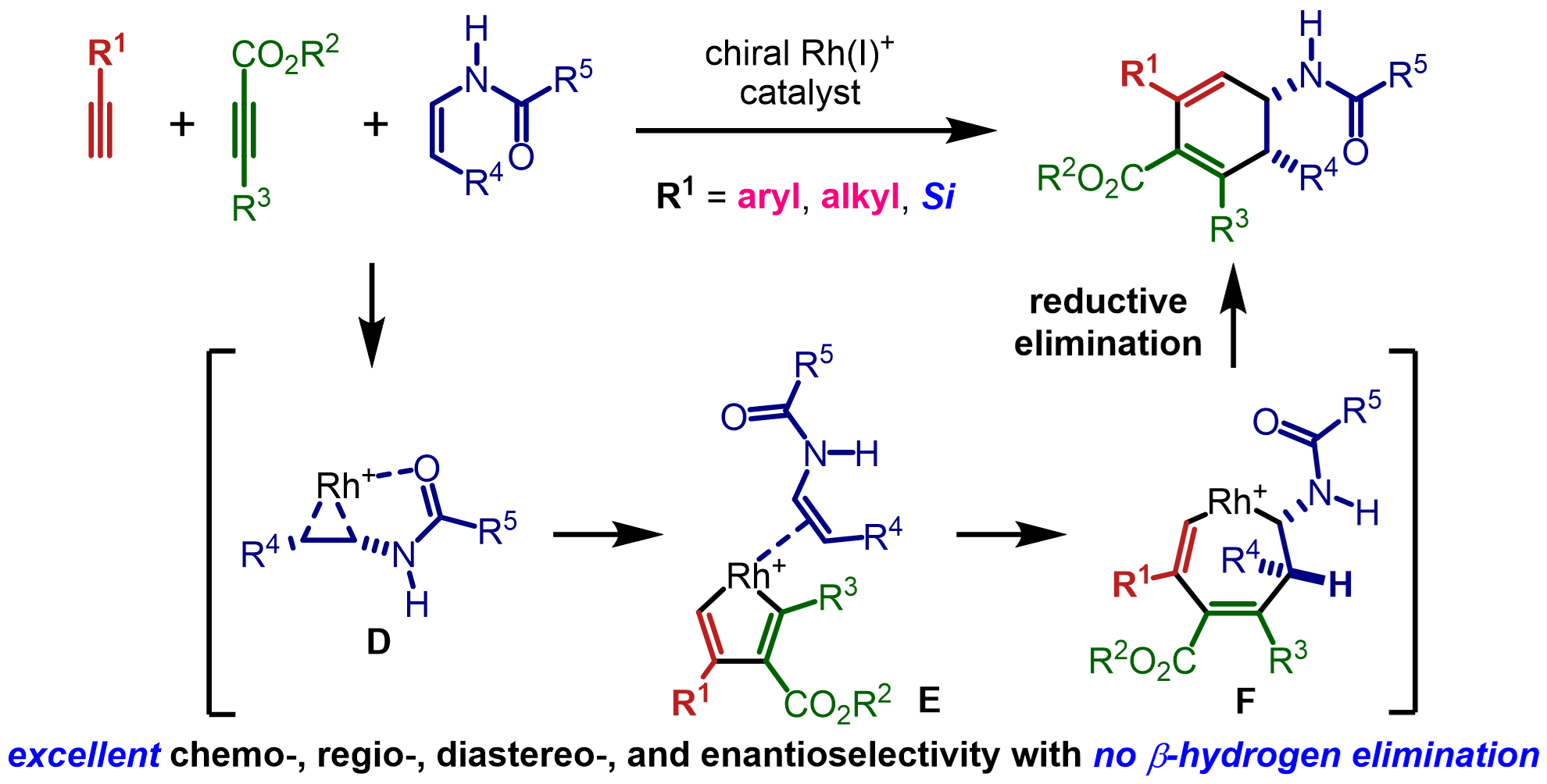
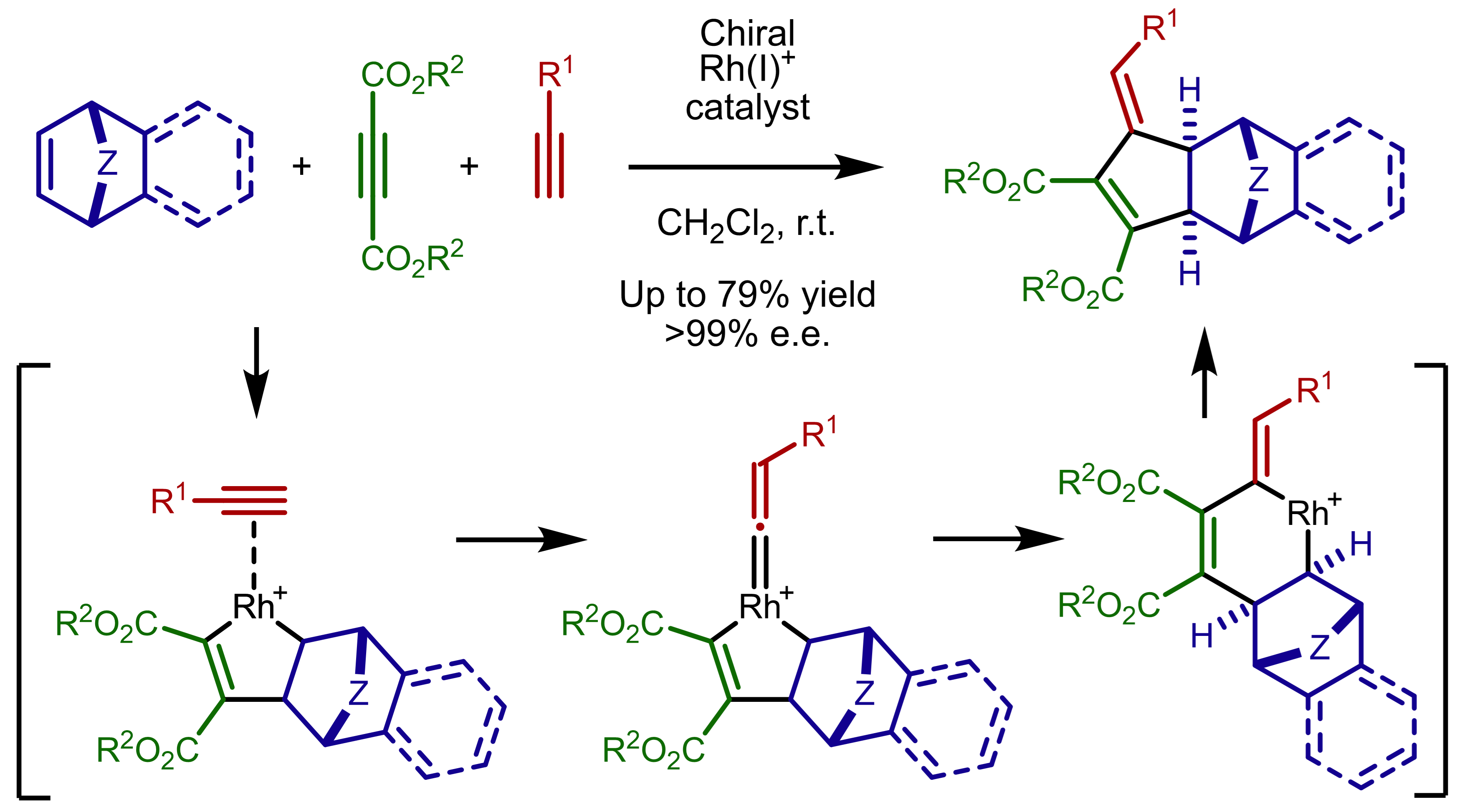
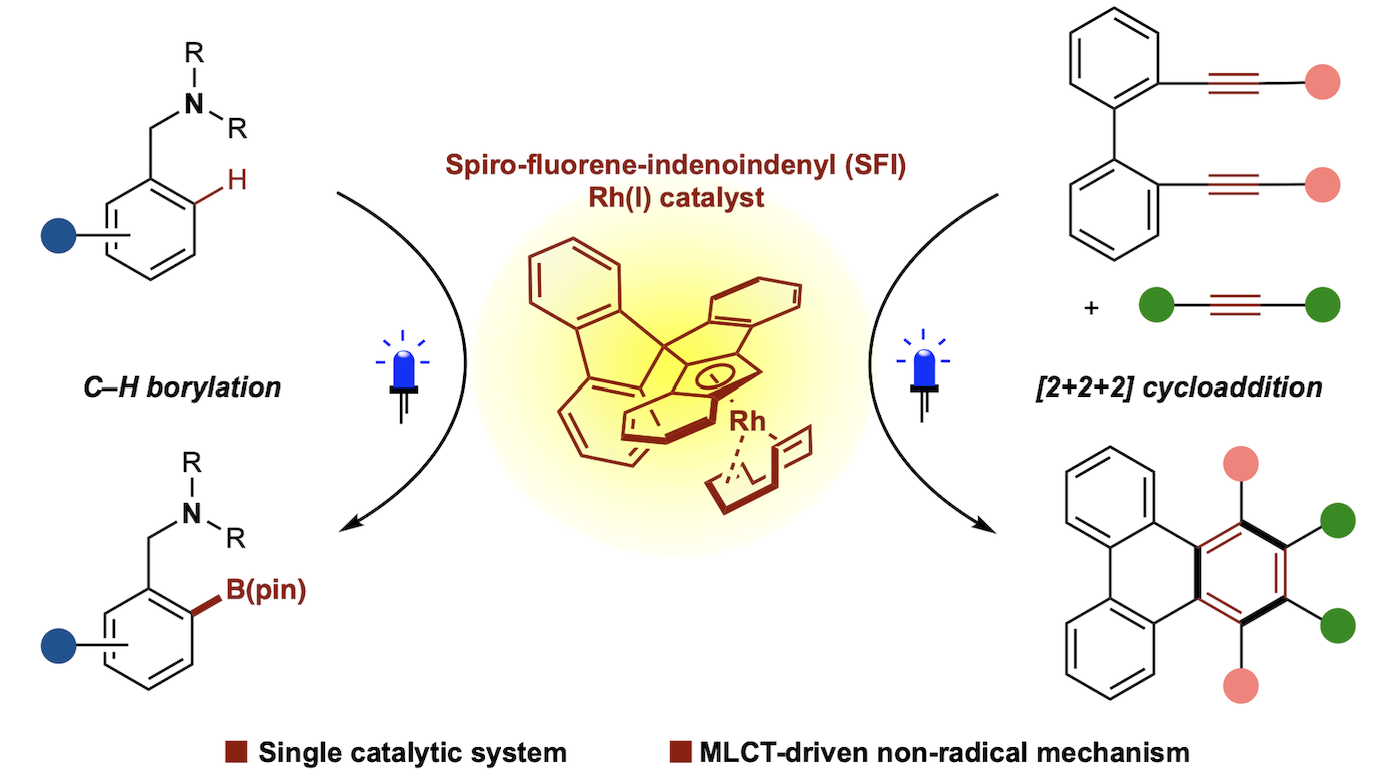
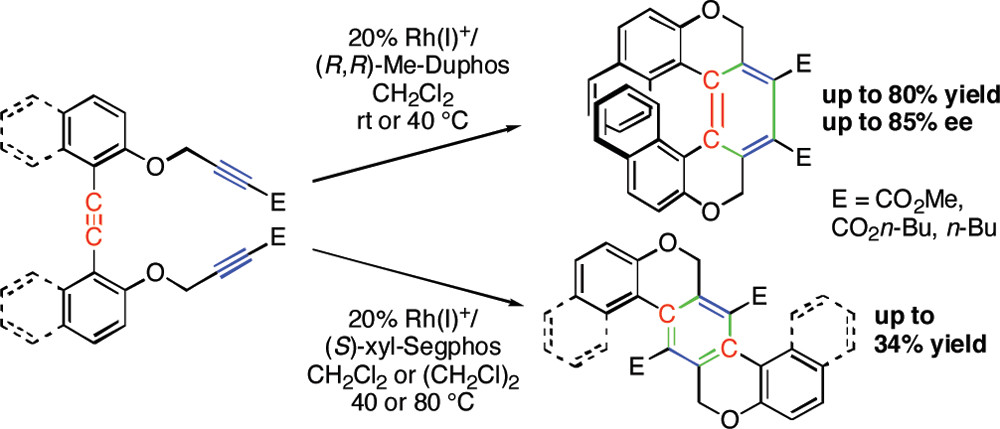
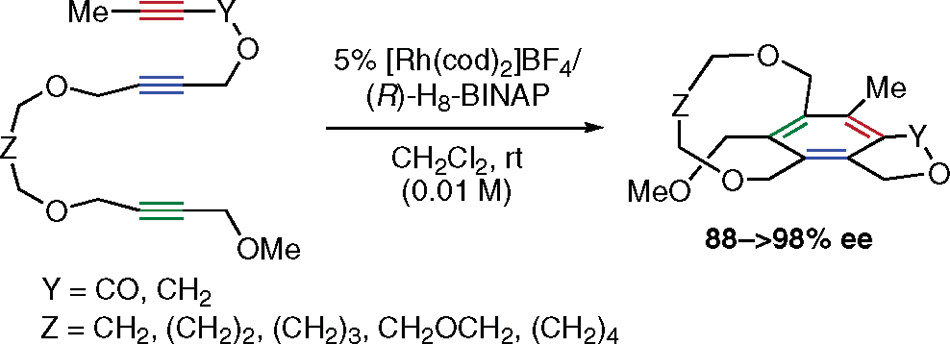
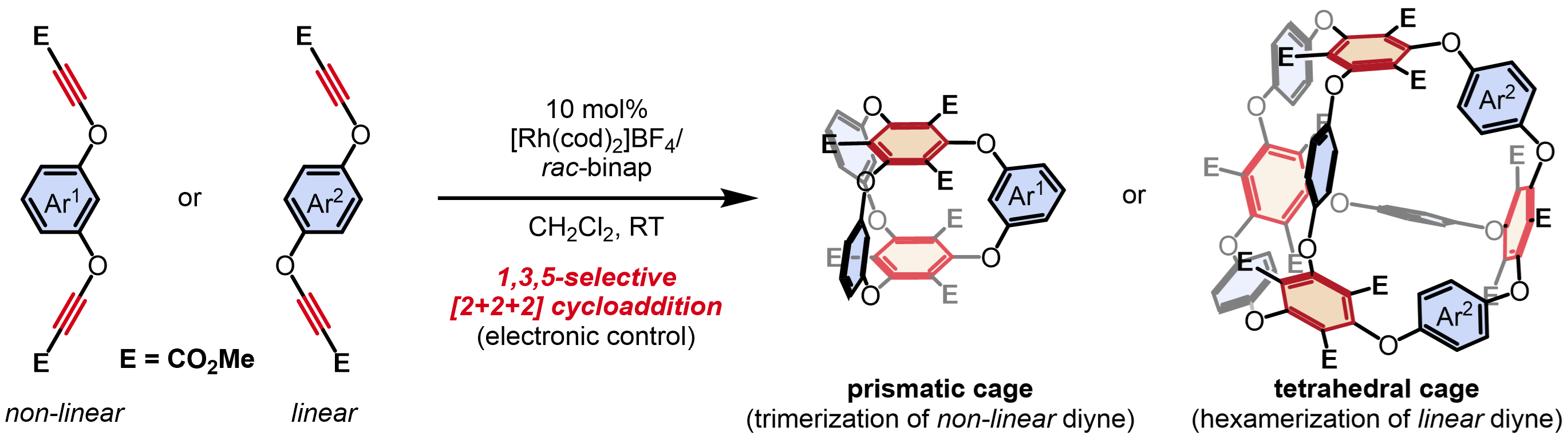
・Rh(I)-catalyzed chemo- and regioselective [2+2+2] cycloaddition reactions ・Rh(I)-catalyzed asymmetric [2+2+2] cycloaddition reactions ・Rh(I)-catalyzed asymmetric [2+2+1] cycloaddition reactionsσ-bond activation reactions
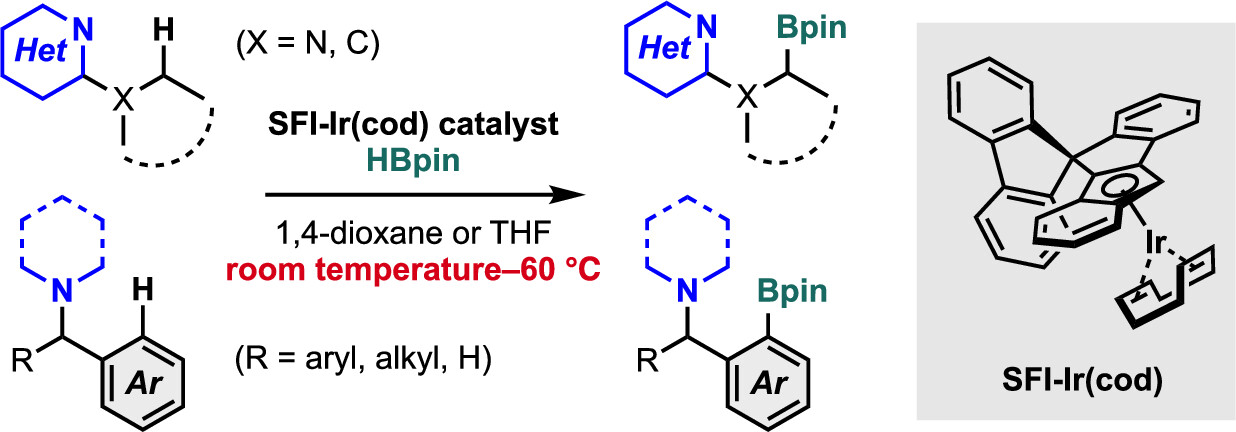
・Rh(I)-catalyzed C-H activation reactions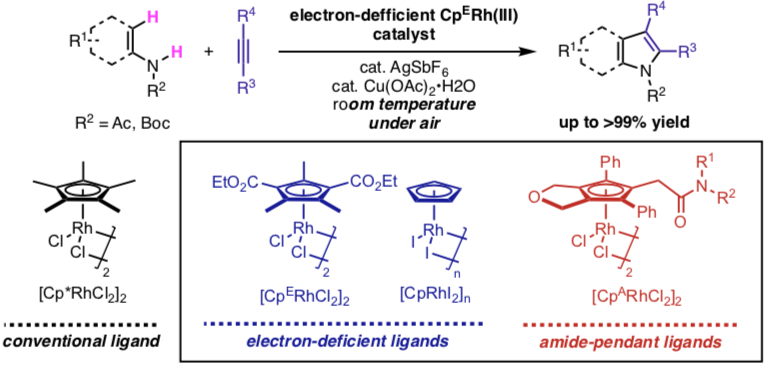
Π-bond activation reactions
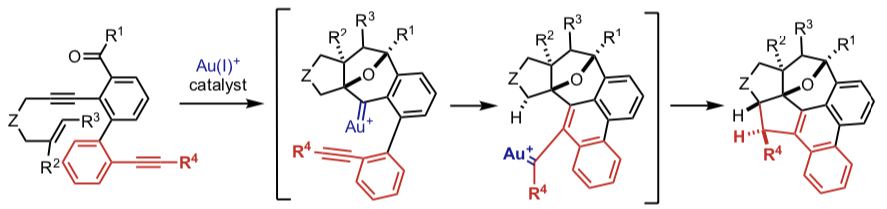
・Au(I)-catalyzed asymmetric annulation reactions ・Au(I)-catalyzed cascade annulation reactions3D Nanocarbon synthesis by [2+2+2] cycloadditions
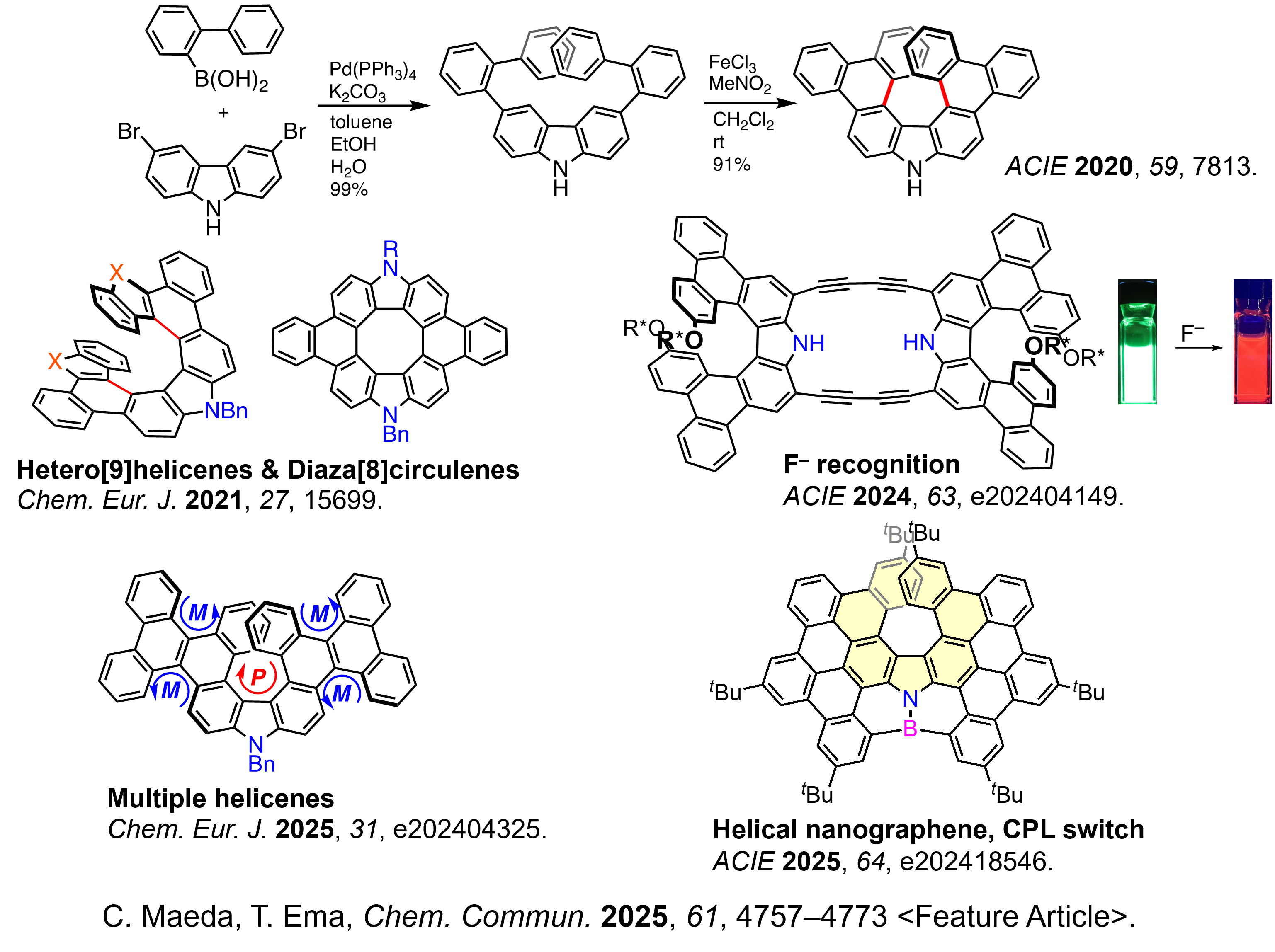
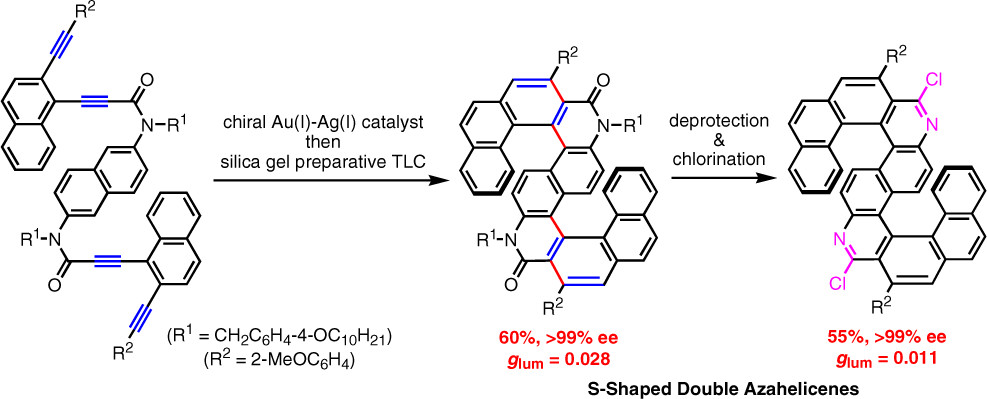
Synthesis of novel azahelicenes