RESEARCH
Research
Our research is based on the development of new reactions facilitated by transition metal catalysts or external stimuli (such as light), and the rational selection and design of ligands and reagents that enable them. Through the development of these new reactions, we are solving long-standing limitations and serious problems in organic synthesis and creating useful and unexplored molecules. In these studies, we are combining theoretical calculations as well as experiments. Theoretical calculations are a powerful method for predicting reaction pathways and physical properties as well as interpreting reaction mechanisms and physical properties. The following are examples of research reported from our group so far.
✓ σ-bond activation reactions
✓ Π-bond activation reactions
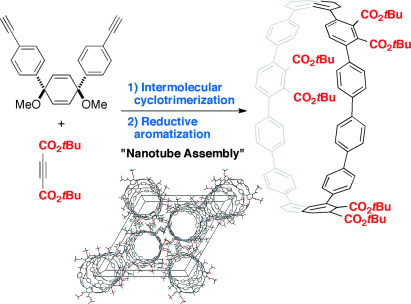
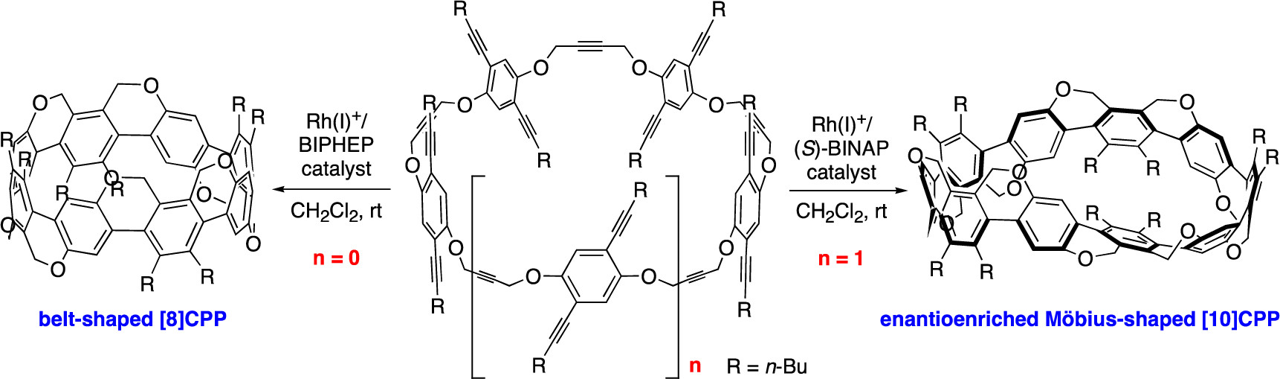
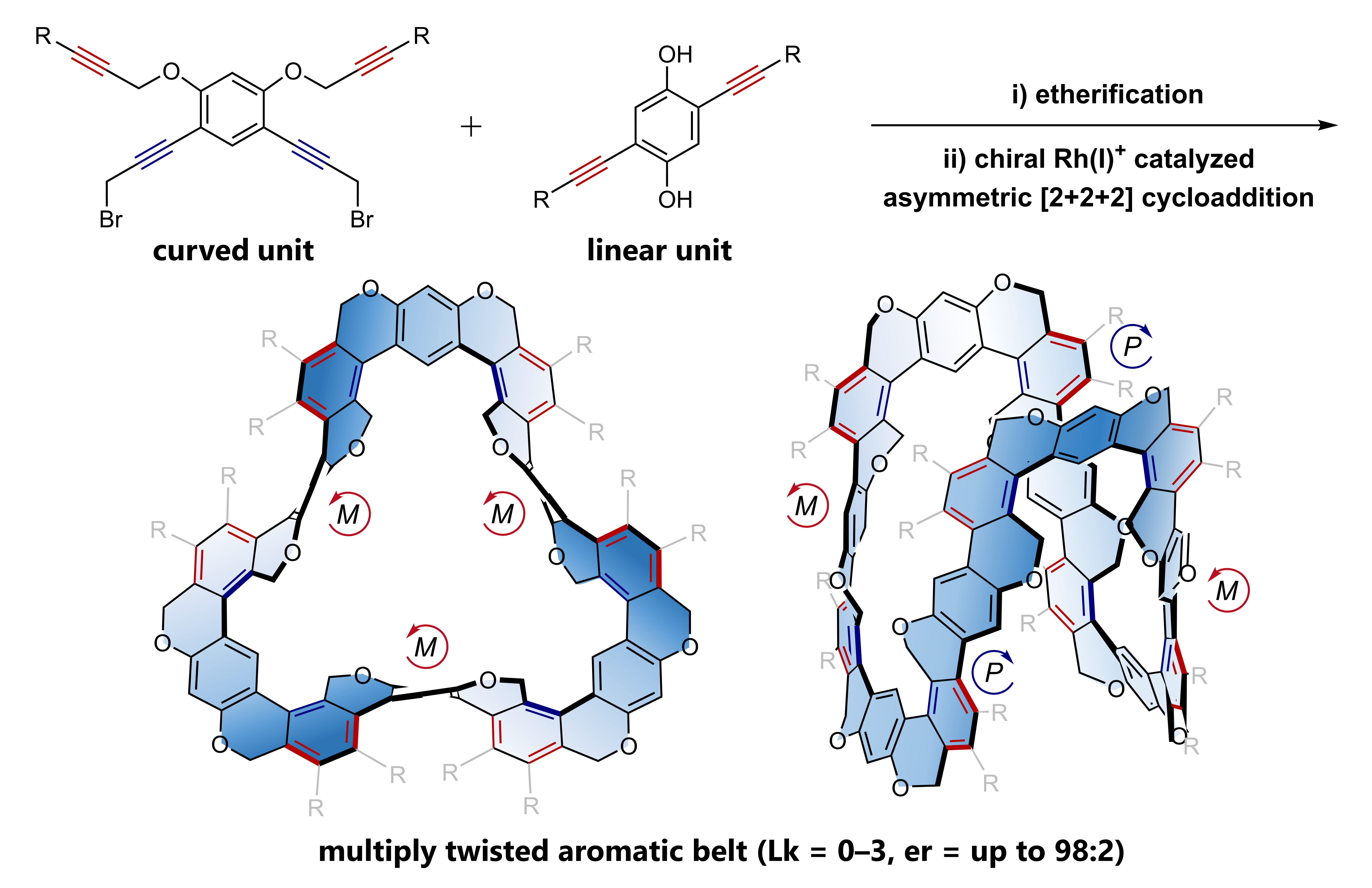
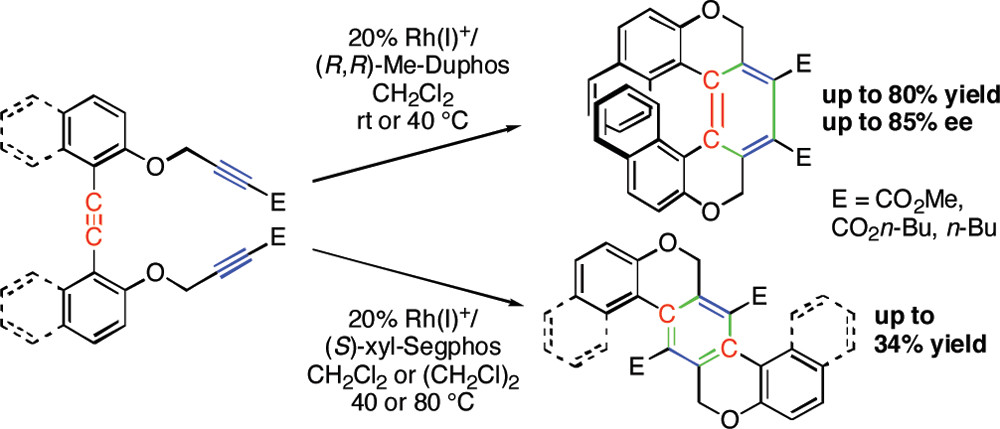
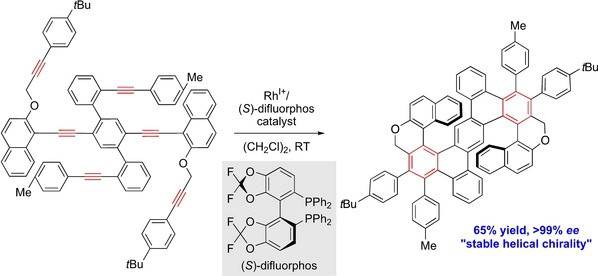
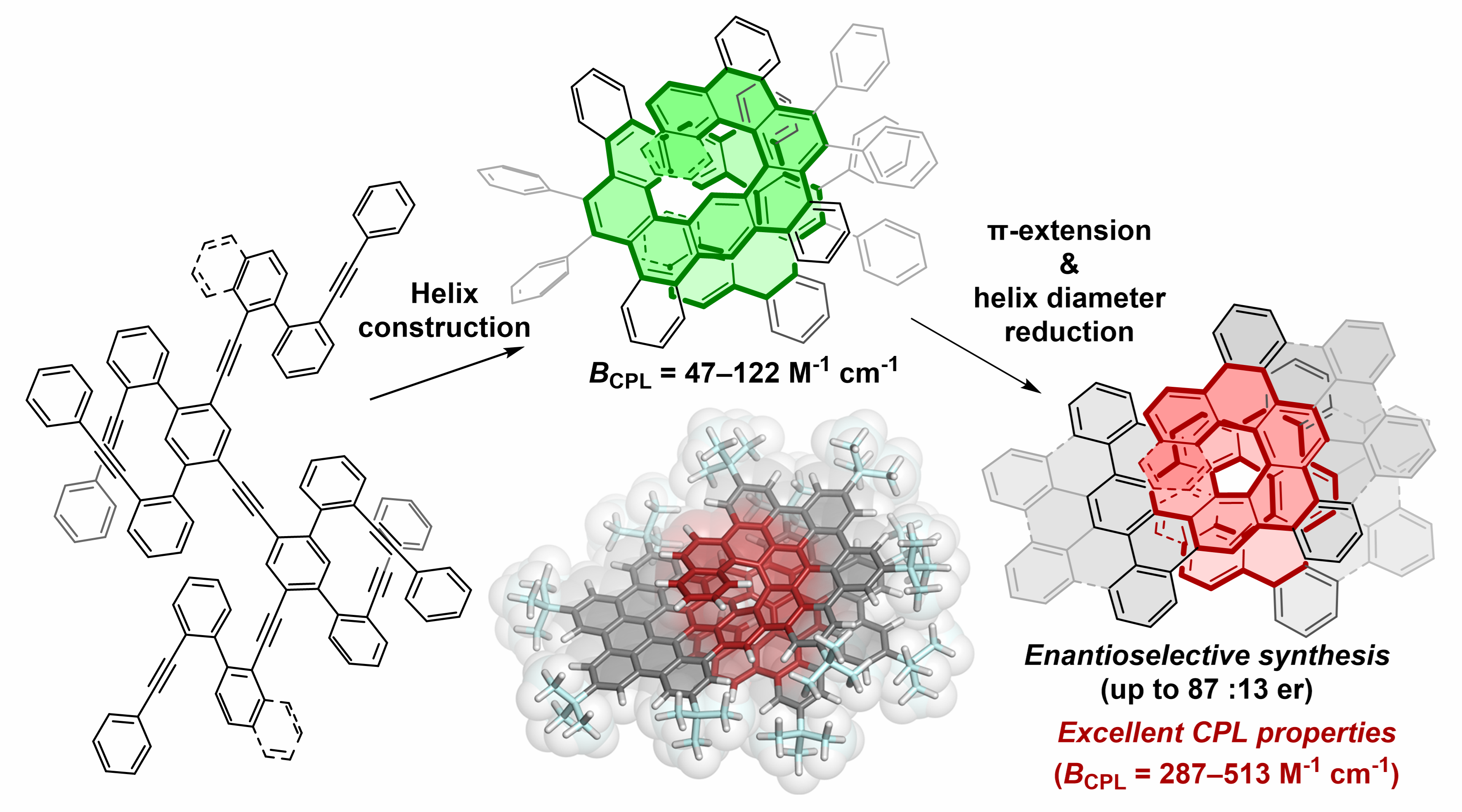
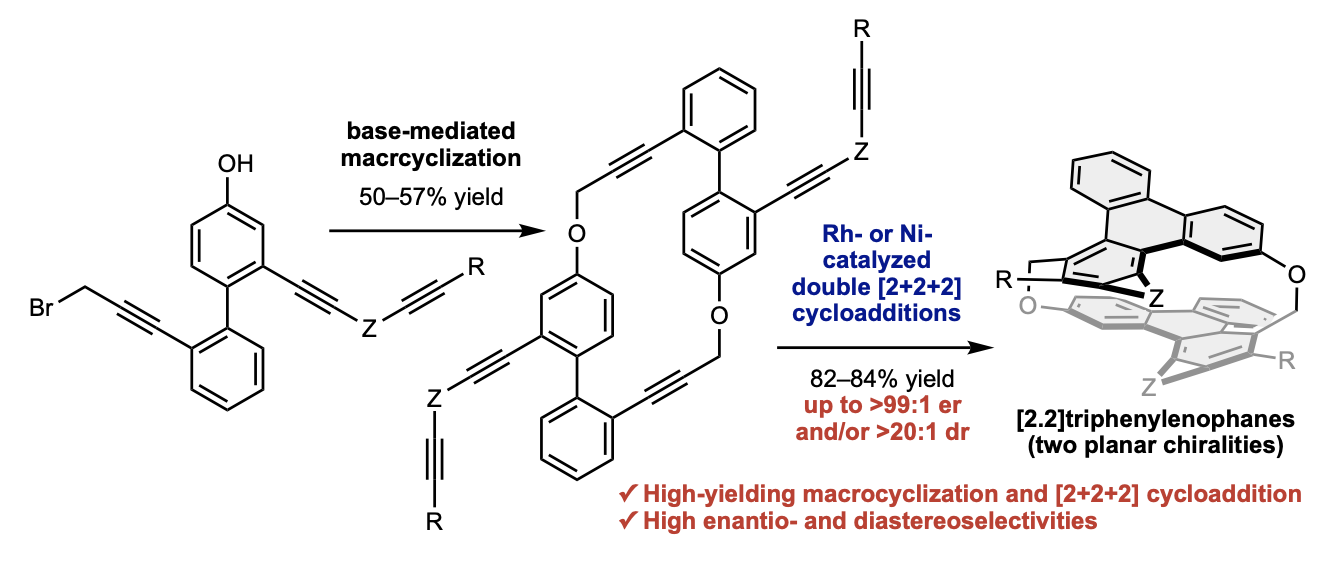
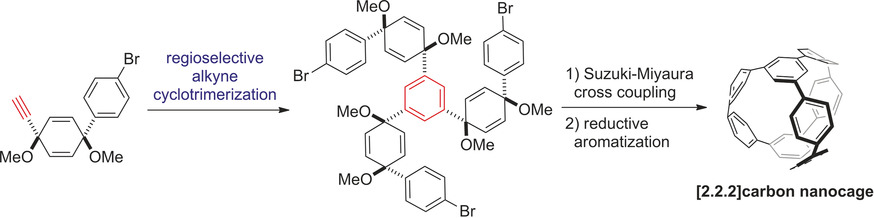
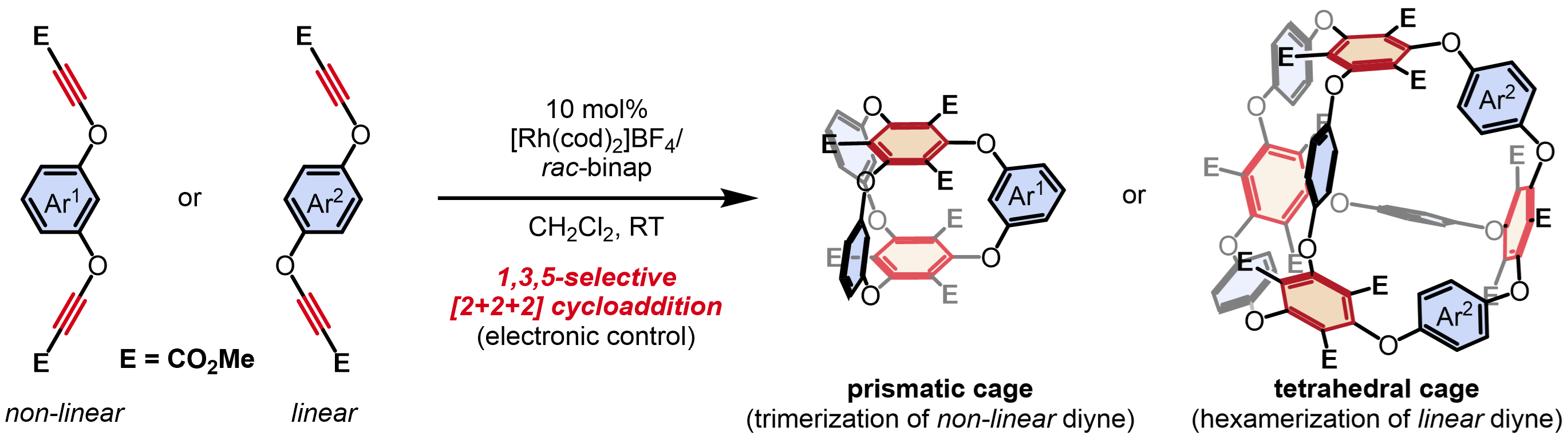
✓ 3D Nanocarbon synthesis by [2+2+2] cycloadditions
Carbon nanorings / Single and multi-helicenes / Cyclophanes / Cages
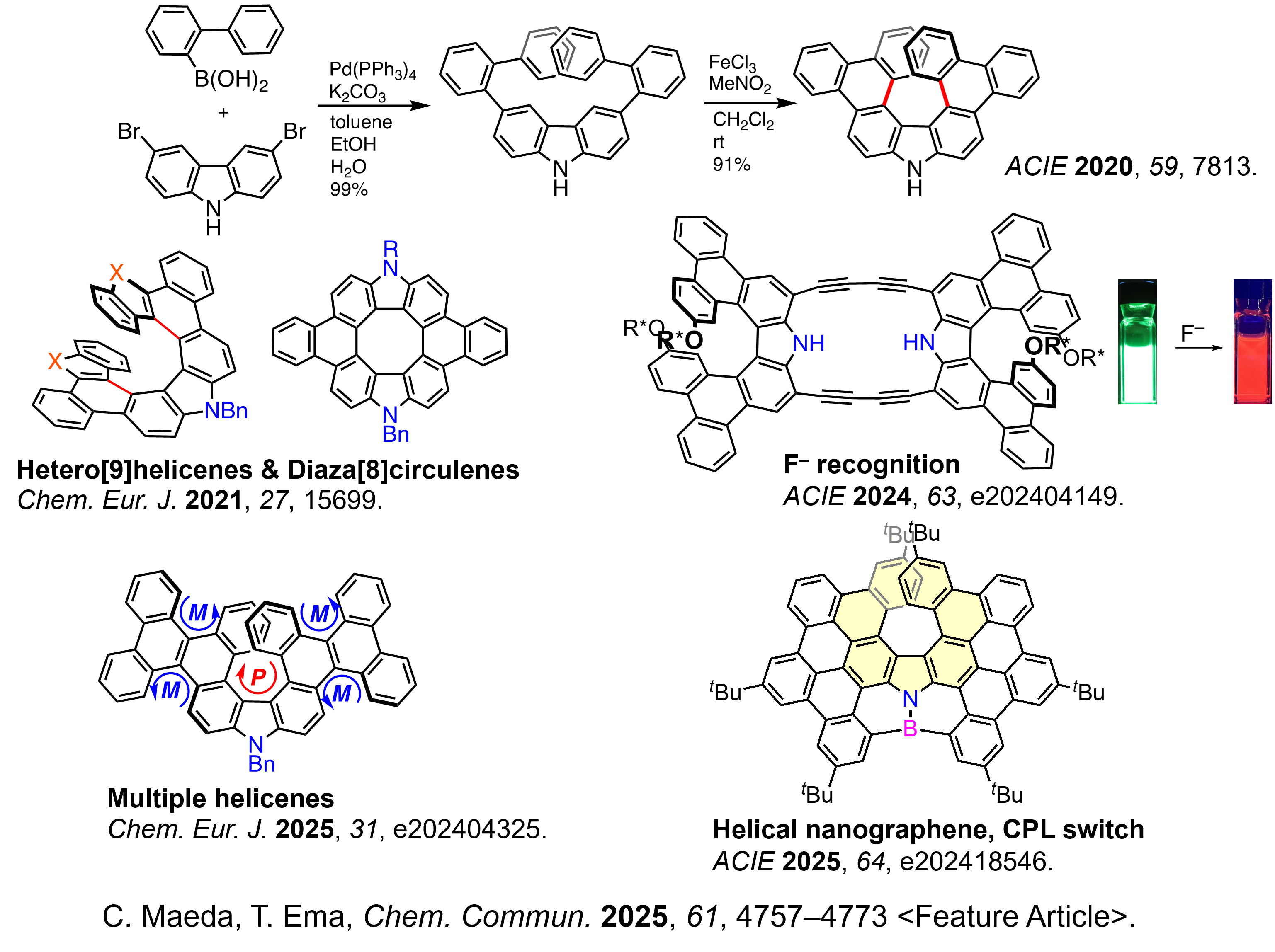
✓ Synthesis of novel azahelicenes
Annulation reactions
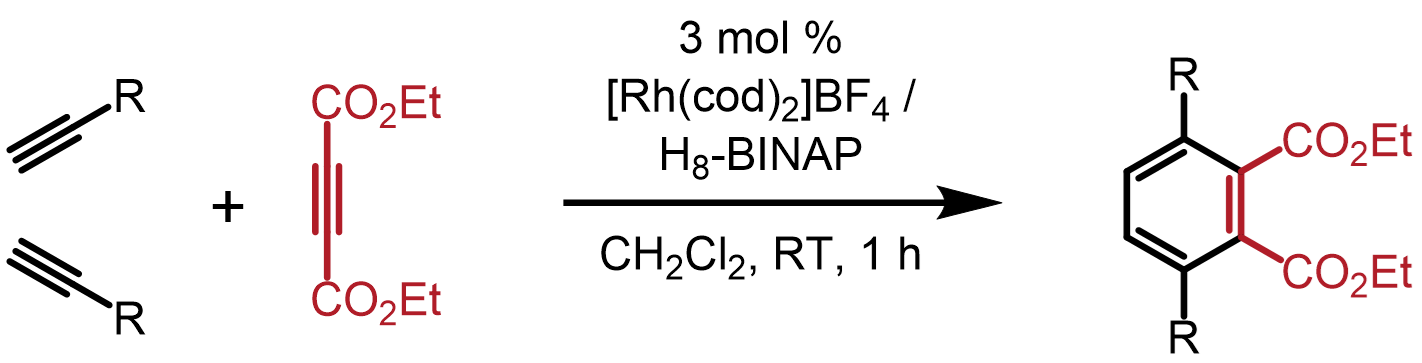
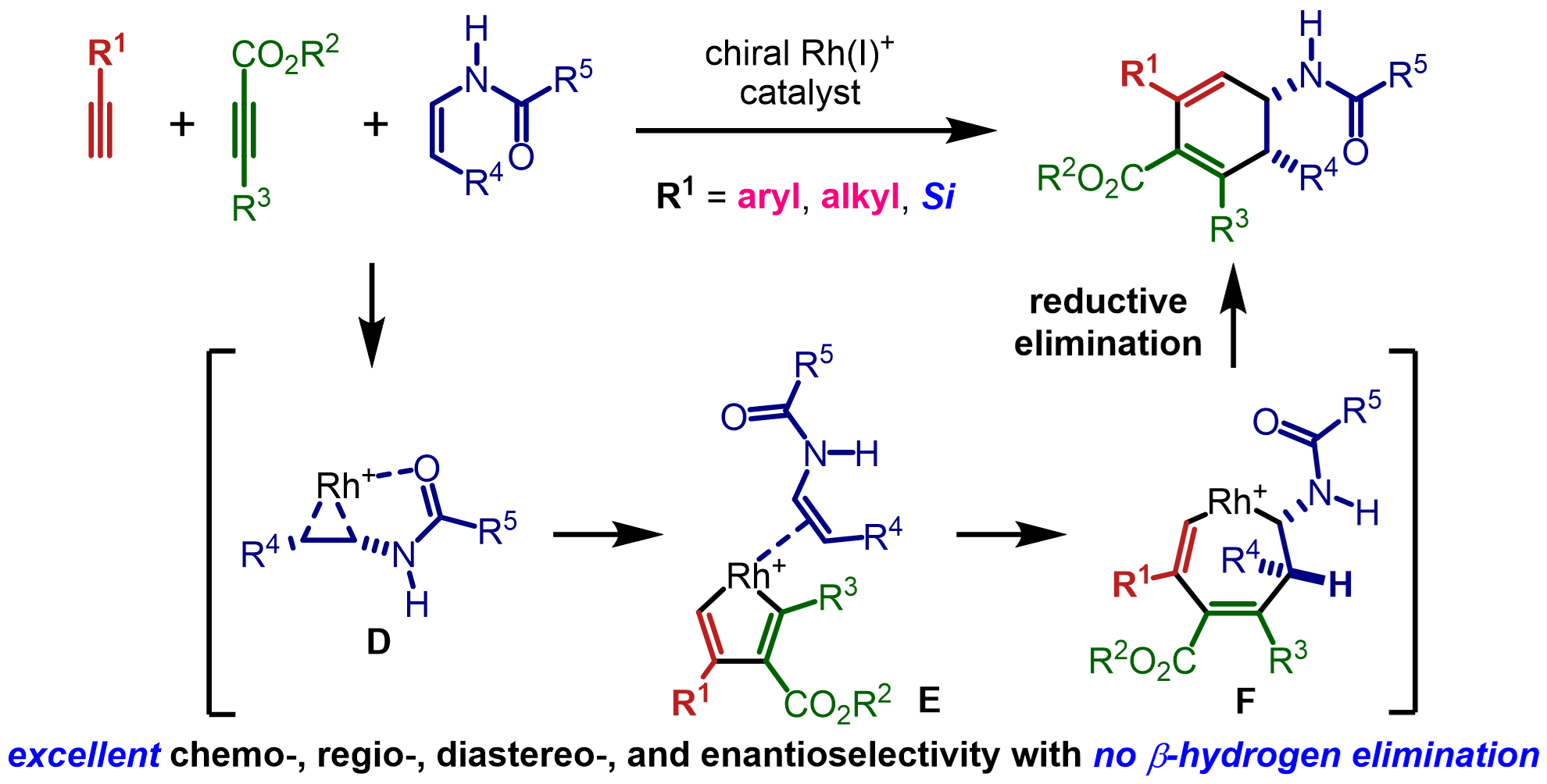
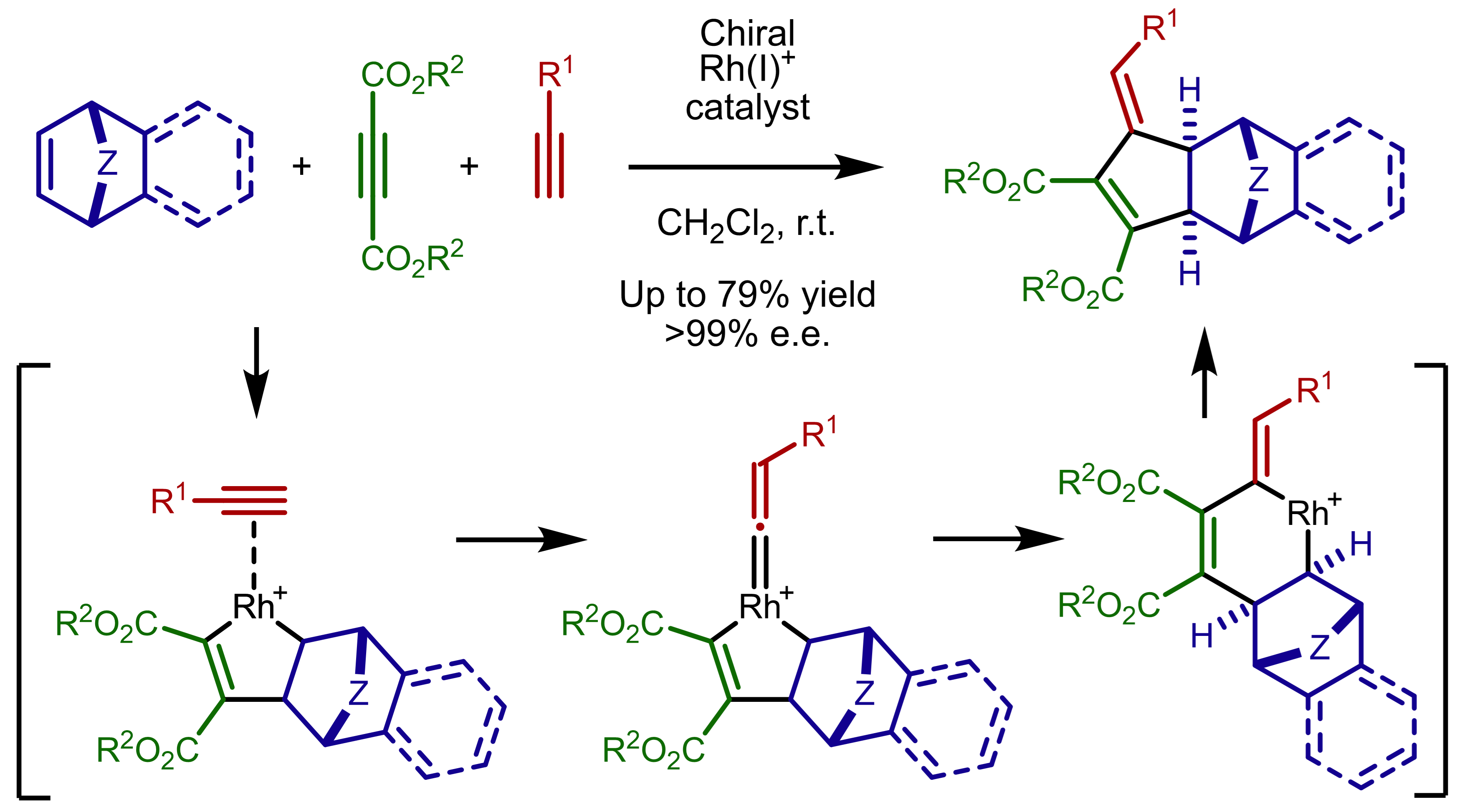
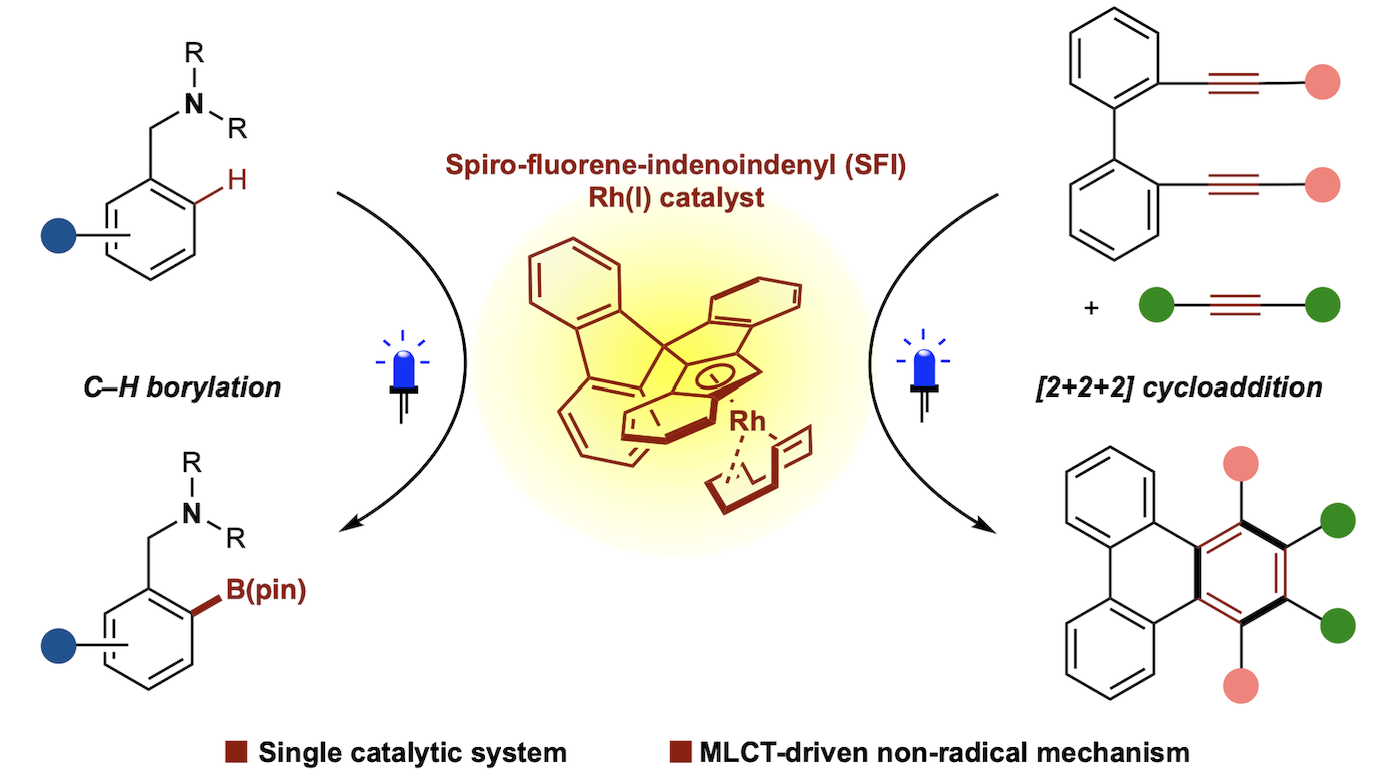
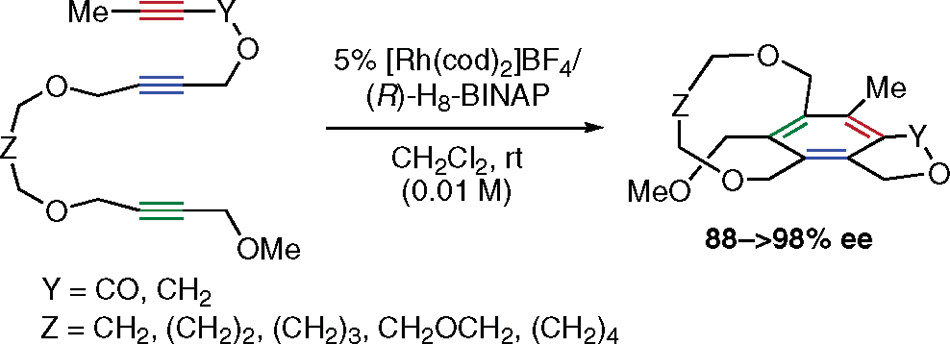
・Rh(I)-catalyzed chemo- and regioselective [2+2+2] cycloaddition reactions ・Rh(I)-catalyzed asymmetric [2+2+2] cycloaddition reactions ・Rh(I)-catalyzed asymmetric [2+2+1] cycloaddition reactionsσ-bond activation reactions
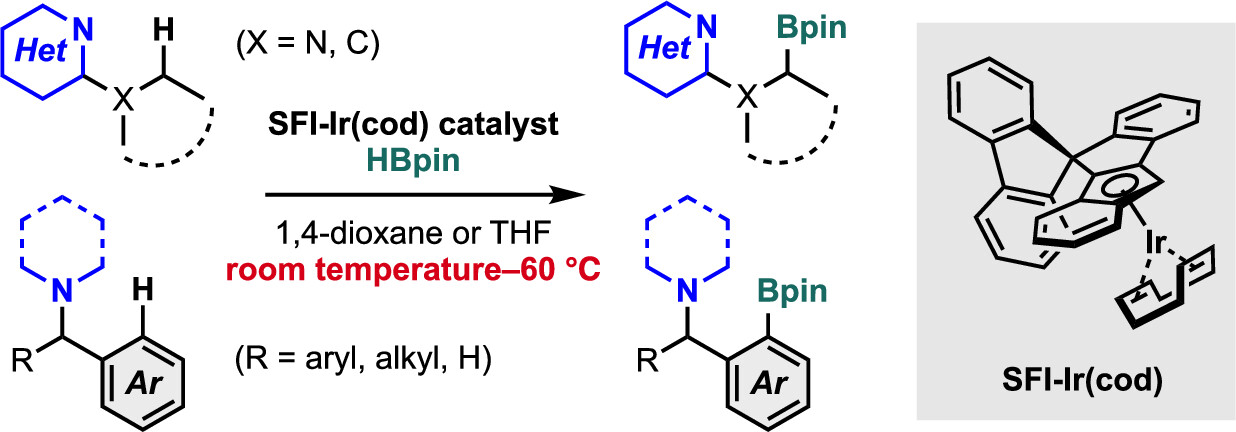
・Rh(I)-catalyzed C-H activation reactions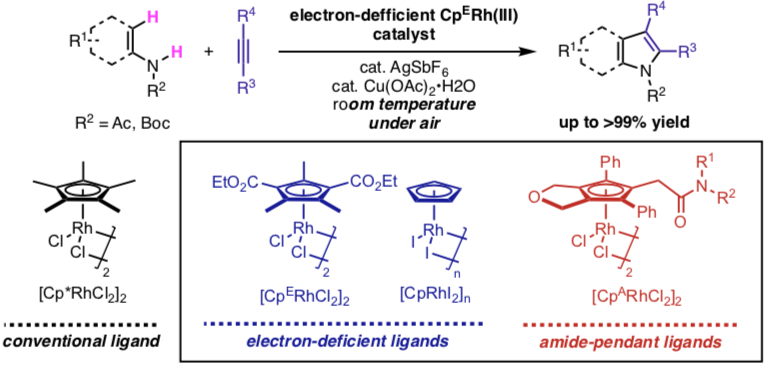
Π-bond activation reactions
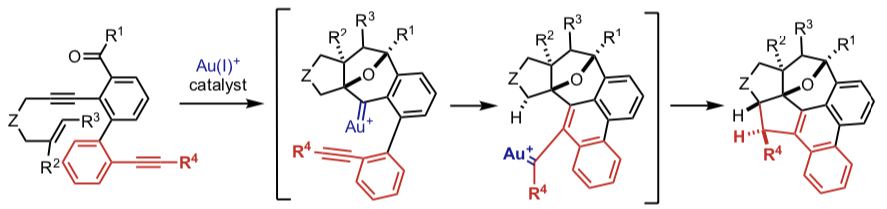
・Au(I)-catalyzed asymmetric annulation reactions ・Au(I)-catalyzed cascade annulation reactions3D Nanocarbon synthesis by [2+2+2] cycloadditions
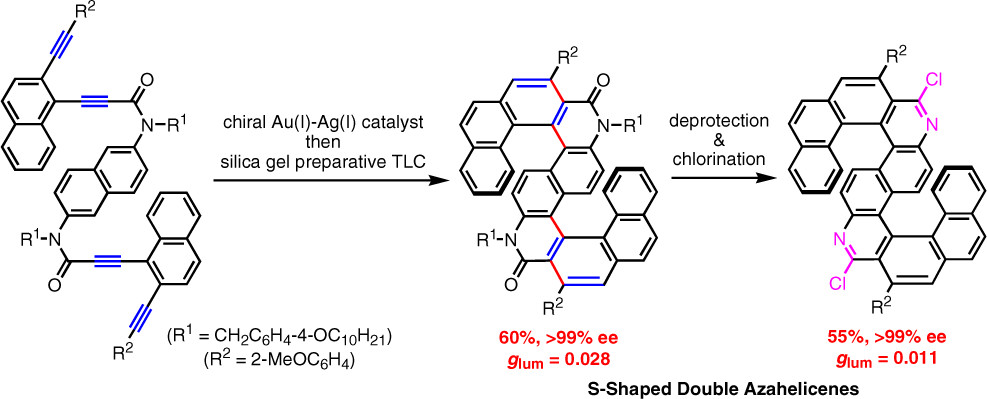
Synthesis of novel azahelicenes